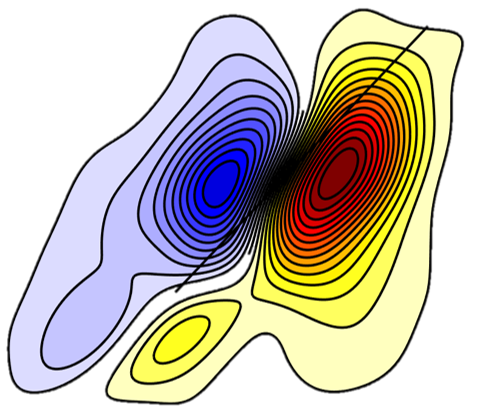
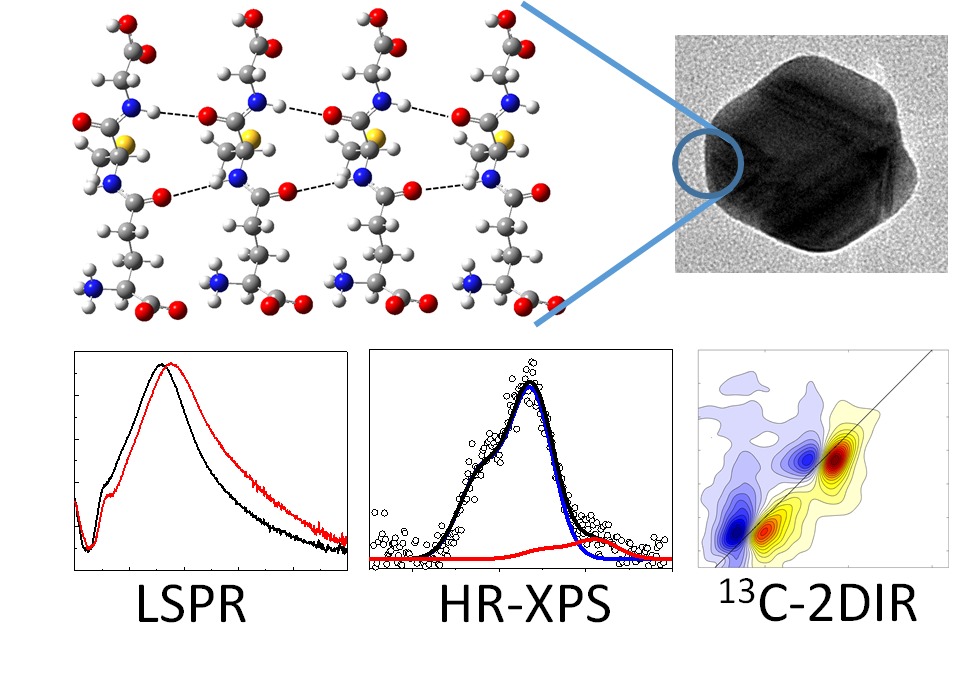
A detailed understanding of the molecular structure in nanoparticle ligand capping layers is crucial for their efficient incorporation into modern scientific and technological applications. Peptide ligands render the nanoparticles as biocompatible materials. Glutathione, a γ-ECG tripeptide, self-assembles into aggregates on the surface of ligand-free silver nanoparticles through intermolecular hydrogen bonding and forms a few nanometer-thick shells. Two-dimensional nonlinear infrared (2DIR) spectroscopy suggests that aggregates adopt a conformation resembling the β-sheet secondary structure. The shell thickness was evaluated with localized surface plasmon resonance spectroscopy and X-ray photoelectron spectroscopy. The amount of glutathione on the surface was obtained with spectrophotometry of a thiol-reactive probe. Our results suggest that the shell consists of ∼15 stacked molecular layers. These values correspond to the inter-sheet distances, which are significantly shorter than those in amyloid fibrils with relatively bulky side chains, but are comparable to glycine-rich silk fibrils, where the side chains are compact. The tight packing of the glutathione layers can be facilitated by hydrogen-bonded carboxylic acid dimers of glycine and the intermolecular salt bridges between the zwitterionic γ-glutamyl groups. The structure of the glutathione aggregates was studied by 2DIR spectroscopy of the amide-I vibrational modes using 13C isotope labeling of the cysteine carbonyl. Isotope dilution experiments revealed the coupling of modes forming vibrational excitons along the cysteine chain. The coupling along the γ-glutamyl exciton chain was estimated from these values. The obtained coupling strengths are slightly lower than those of native β-sheets, yet they appear large enough to point onto an ordered conformation of the peptides within the aggregate. Analysis of the excitons’ anharmonicities and the strength of the transition dipole moments generally is in agreement with these observations.